Acoustic Soil
Barely audible to human ears, healthy soils produce a cacophony of sounds in many forms — a bit like an underground concert of bubbles and clicks.
Special recordings made by Flinders University ecologists in Australia show this chaotic mixture of soundscapes can be a measure of the diversity of tiny living animals in the soil, which create sounds as they move and interact with their environment. This new field of research aims to investigate the vast, teeming hidden ecosystems where almost 60 percent of the Earth’s species live
“Restoring and monitoring soil biodiversity has never been more important,” said lead researcher Jake Robinson. “Although still in its early stages, ‘eco-acoustics’ is emerging as a promising tool to detect and monitor soil biodiversity and has now been used in Australian bushland and other ecosystems in the UK. The acoustic complexity and diversity are significantly higher in revegetated and remnant plots than in cleared plots, both in-situ and in sound attenuation chambers. The acoustic complexity and diversity are also significantly associated with soil invertebrate abundance and richness.”
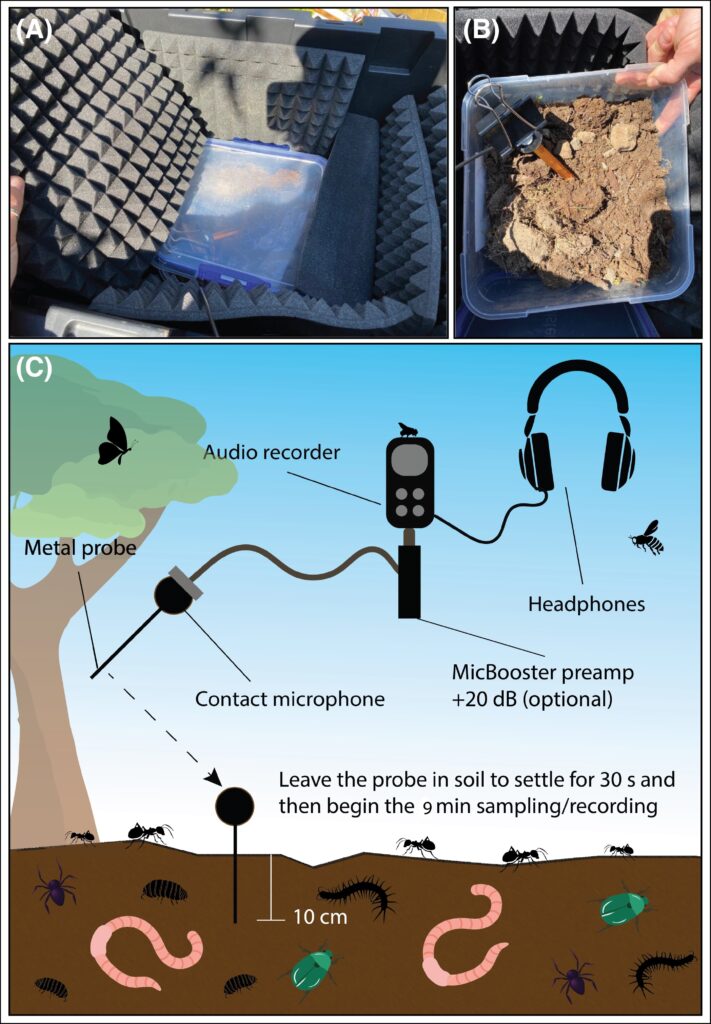
The study compared results from acoustic monitoring of remnant vegetation to degraded plots and land that was revegetated 15 years ago. The passive acoustic monitoring used various tools and indices to measure soil biodiversity over five days in the Mount Bold region in the Adelaide Hills in South Australia. A belowground sampling device and sound attenuation chamber were used to record soil invertebrate communities, which were also manually counted.
“It’s clear acoustic complexity and diversity of our samples are associated with soil invertebrate abundance — from earthworms, beetles to ants and spiders — and it seems to be a clear reflection of soil health,” said Robinson. “All living organisms produce sounds, and our preliminary results suggest different soil organisms make different sound profiles depending on their activity, shape, appendages and size. This technology holds promise in addressing the global need for more effective soil biodiversity monitoring methods to protect our planet’s most diverse ecosystems.”
Changing Watering Practices to Improve Plant Health
Some people believe that talking to your plants makes them thrive. While there’s limited scientific support for sound improving plant health, there’s a growing amount of evidence about the benefits of mechanical stimulation, like touch, wind or rain.
Researchers reporting in Journal of Agricultural and Food Chemistry examined the impact of watering practices on tomato plants. They found that the size of the water droplets affected plant growth and resistance to pests and pathogens.
The researchers sprayed tomato plants with water twice daily and compared the effects of small droplets (200 micrometers, about the size of a computer monitor pixel) and large droplets (1,000 micrometers, about the diameter of a pinhead) versus no spray. They covered the soil with a barrier to ensure the spray didn’t influence how much water the roots received.
Structural observations revealed:
- Tomato plants sprayed with large water droplets were shorter and more compact than other groups.
- There were minimal visible differences between plants receiving small droplets and those receiving no spray.
- Fruit yield and quality were similar among the three groups.
Despite there being not change in fruit yield or quality, metabolic analysis revealed that tomato plants sprayed with large water droplets had:
- Significant changes in hormones involved in plant defenses, resulting in increased resistance to destruction by moth larvae or gray mold compared to plants that weren’t sprayed.
- Higher levels of defense-mediating chlorogenic acid in their leaves compared to the other plant groups.
- Reduced emissions of volatile organic compounds compared with plants receiving no spray, making them less attractive to egg-laying moths and resulting in 74 percent fewer eggs on the leaves.
Given these results, the researchers suggested that continued developments in water spray technologies and droplet atomization could improve agricultural practices, making farming greener and more efficient.
A Gentle and Versatile Robotic Gripper for Efficient Crop Harvesting

Conventional robotic grippers struggle to adapt to complex shapes, sizes and textures, such as those found in crops. This has created a demand for more adaptable robotic grippers that can be used in agriculture.
In a new study, researchers introduced an innovative soft robotic gripper named ROtation-based Squeezing grippEr (ROSE) and optimized its unique wrinkling-based grasping mechanism using simulations. ROSE’s soft-yet-secure grasp can make it a vital tool for agriculture.
Robotic grippers have become essential across many industries, including manufacturing, packaging, and logistics, mainly for pick-and-place tasks. Recently, the demand for robotic grippers has also expanded into agriculture, where they are used for harvesting and packaging tasks. However, conventional robotic grippers struggle with the unique shapes, properties, and delicate nature of different crops.

Robotic grippers that are made of soft materials have emerged as a potential solution to these problems. However, current methods for adapting these grippers to complex geometries rely on complex control and planning generated by data-based models. These models require a large amount of data, limiting their general applicability. Additionally, integrating a sensory system into their soft body requires complex designs and sophisticated fabrication methods.
“ROSE takes inspiration from the blooming states of a rose to generate grasping action,” explained lead researcher Van Anh Ho. “It offers a simpler approach to real-farm harvesting by gently grasping objects using a unique ‘wrinkling’ phenomenon. Unlike conventional grippers, ROSE doesn’t require complex control and planning strategies to adapt to various agricultural products with diverse shapes, sizes and textures.”
The team employed a simulation model to fully understand and optimize the grasping mechanism of ROSE. The study was published in the International Journal of Robotics Research.
ROSE consists of an isolated cup-shaped chamber formed by two thin, soft elastomer layers, with a separation between the interior and outer layers. Rotating only the inner layer using an external motor produces a deformation in the layers. Specifically, this twisting motion of the inner layer results in a strain mismatch between the outer and inner layers, resulting in the formation of a series of wrinkle-like inward folds, a process termed “wrinkling.” This unique mechanism shrinks the central space in ROSE, which allows it to gently grasp any object present within this central space.
The researchers demonstrated the practical applications of ROSE in agriculture by using it to harvest strawberries and mushrooms. ROSE achieved high success rates in picking up these crops in multiple trials, regardless of whether they were firm or soft. It also succeeded in picking up a clump of mushrooms without breaking any piece, provided the clump size fit within the grasping space.
Imaging Pathogens on Lettuce Leaves in Real-Time
Plant diseases such as downy mildew pose significant threats to crops. Dutch researchers from Delft University of Technology have developed a new method to monitor infections in plants in real-time, without the need to destroy the plants. The findings were published in Nature Communications.
Farmers growing lettuce prefer varieties that are resistant to various diseases, including downy mildew, a common plant disease that causes yellow or brown spots on the upper surfaces of leaves. Delft scientists investigated downy mildew infections in lettuce, a plant species where such infections are typically only visible in its later stages.
“There are indeed lettuce varieties that are resistant to downy mildew, but similar to the coronavirus, the disease continually evolves into new variants that can still infect resistant plants,” explained physicist Jos de Wit. “This forces scientists and breeders into a constant race to develop new resistant crops in response to evolving diseases.”
To cultivate crops like lettuce that can better withstand diseases, the researchers developed a method that allows for imaging common plant infections, without killing the plant and significantly faster than conventional microscopy. “Until now, researchers had to kill a plant for each step in the process, stain it, and then examine it under a microscope,” said researcher Jeroen Kalkman. “With this new imaging technique, we can track how a disease develops in a living plant in real-time.”
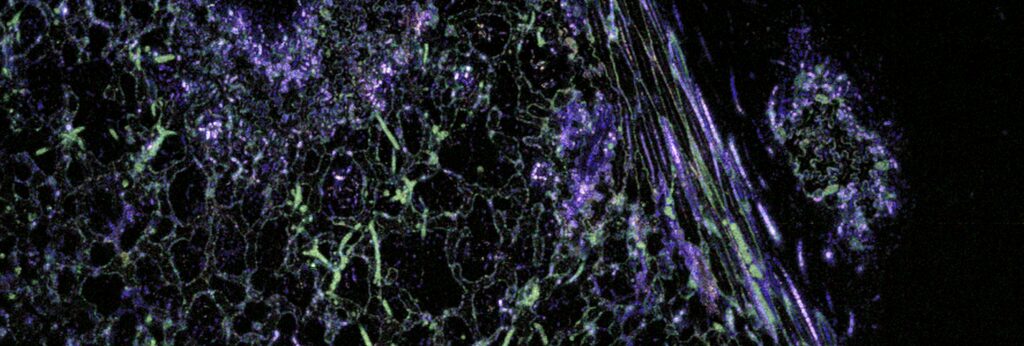
“The technique we used is called dynamic optical coherence tomography (dOCT),” said De Wit. “It involves emitting light and measuring the time it takes for that light to reflect back — similar to ultrasound but with light instead of sound. In just one and a half seconds, we can capture around 50 to 100 images of an infected lettuce leaf. We can effectively map plant diseases with dOCT because the pathogens move more than the plant cells. By assigning colors to areas with more movement, we can generate a strong contrast between the pathogen and the plant. Without dOCT, the disease would only be visible at a much later stage.”
The researchers have demonstrated that their instrument also works for crops such as radishes and peppers that carry parasitic roundworms. Further research is needed, however, to make this technique a user-friendly tool for biologists without a technical background.














