Understanding iron’s mechanisms in productive soils
Iron (Fe), a ubiquitous element in the Earth’s crust, is a foundational building block in the world of agriculture.
From a scientific perspective, it’s not just the presence of iron that governs the growth and productivity of crops — it’s iron’s chemical state. The significance of biological and nonbiological processes that govern how iron cycles between available and unavailable forms is often overlooked. Consequently, understanding how soils and plants influence the optimal uptake and incorporation of iron — and, more importantly, how to manage it — can help maximize your crops’ health and productivity.
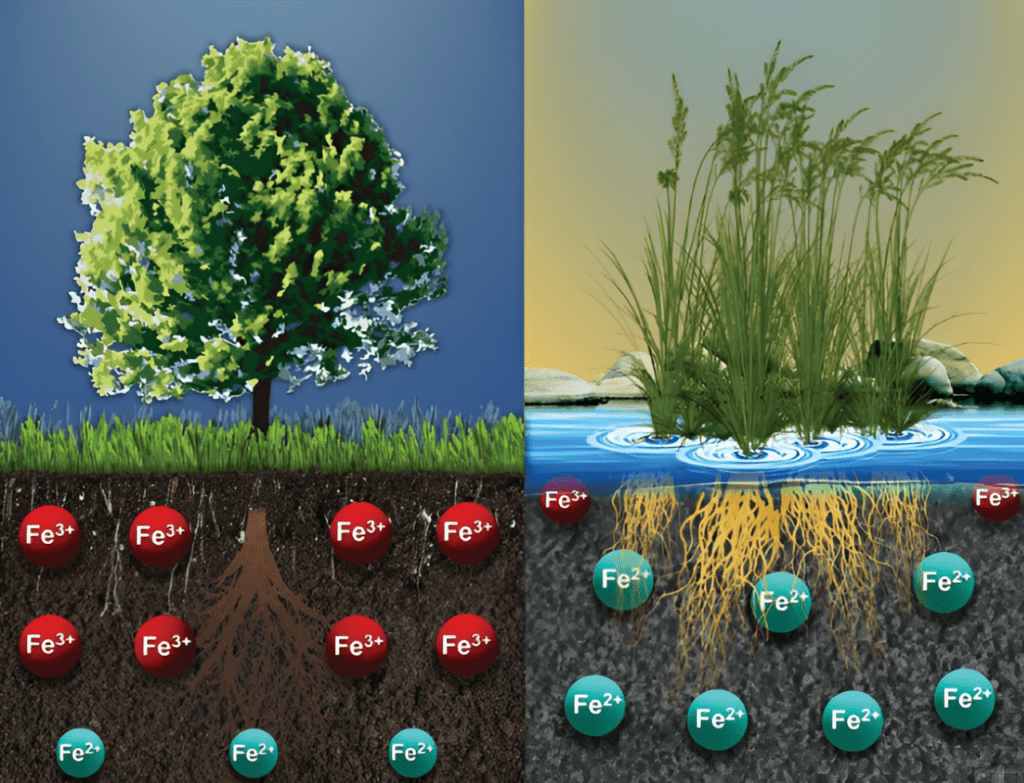
Soil iron predominantly occurs in two states: the reduced form (Fe2+, or ferrous iron) and the oxidized form (Fe3+, or ferric iron). Both are governed by the soil’s acidity or alkalinity (pH) as well as its redox potential, or how oxygenated the environment is. In fact, oxygen levels are just as critical as pH in soil systems, especially in areas that experience heavy rainfall, high water tables, or impermeable hardpans. In conditions where oxygen is bountiful, the equilibrium tips in favor of oxidized, less-available forms of iron; in water-saturated soils where oxygen is sparse, the scale leans toward the reduced, soluble form, underscoring the vital influence of soil redox conditions on iron’s bioavailability to plants.
Iron Uptake: Utilization and Pathways Inside the Plant
Plants possess a marked preference for the ferrous form of iron, primarily due to its higher solubility and, hence, easier uptake. This form is abundant in waterlogged conditions; however, in most agricultural soils, the less-soluble ferric form of iron is predominant.
To bridge this gap, plants release specialized molecules called phytosiderophores that chelate a range of micronutrients, including iron. These novel biochemical adaptations bind and reduce ferric iron to its ferrous form, making it available for plant uptake.
In acidic soil environments (pH < 7), plants can also release smaller organic acids as part of their root exudates, such as citrate, oxalate, and malate. These organic acids serve as chelators and can even protect the plant from pH-induced impacts, such as aluminum (Al) toxicity, by binding with Al ions prevalent under these acidic conditions.
In neutral pH environments (pH 7), plants release a more balanced mixture of organic acids, amino acids, and sugars for iron chelation and as biochemical signalers to interact with the soil microbiome and aid nutrient uptake.
In alkaline or basic soil environments (pH > 7), where iron deficiency is likely to occur, plants may rely more on exudates like phytosiderophores for nutrient acquisition. Some plants also release phenolic compounds like coumarins, which can enhance iron availability in alkaline soils. Additionally, certain plants can alter soil pH directly by releasing protons (H+ ions) to acidify their immediate environment and make nutrients more available.
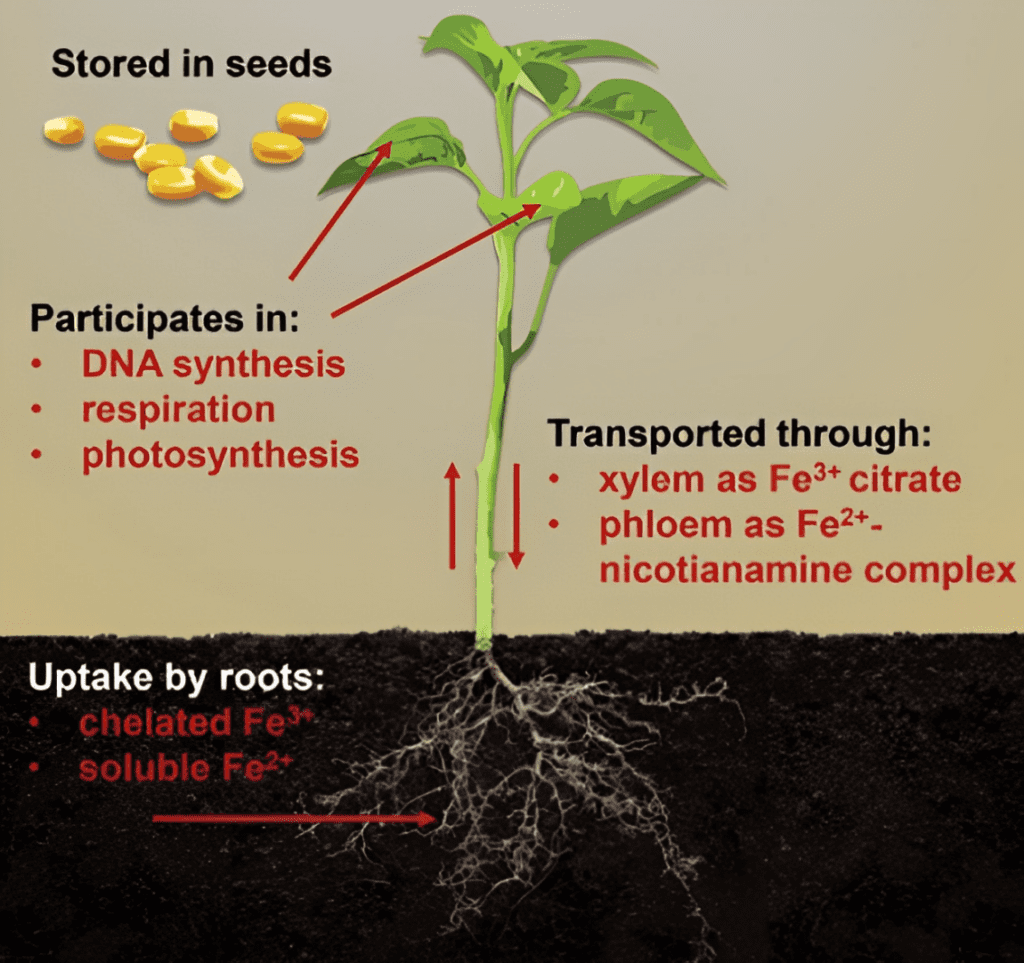
After iron is taken up by the root, it is transported to the shoot through the xylem, primarily in the Fe3+-citrate form. Once absorbed, iron undergoes various transformations inside the plant. Despite being a micronutrient, iron plays integral roles in various processes. It is essential for photosynthesis, chlorophyll synthesis, respiration, DNA synthesis, and serves as a critical cofactor for several enzymes. Thus, iron is vital for energy transfer, metabolism, and nitrogen fixation.
The Iron Balance: Too Little Versus Too Much
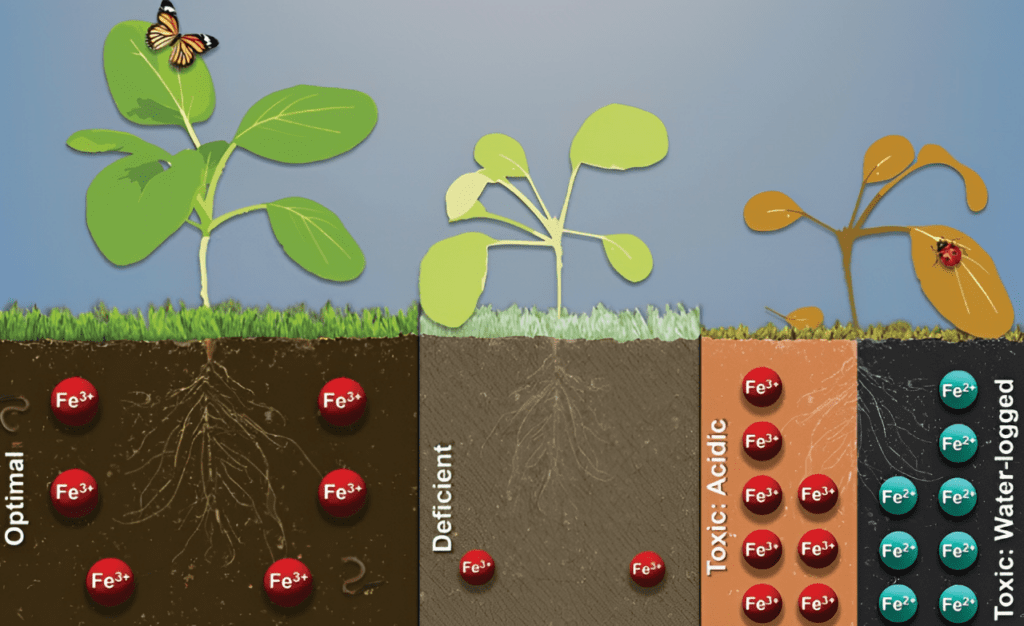
Both iron deficiency and toxicity can pose problems for plants. Iron deficiency typically manifests as interveinal chlorosis, where leaves turn yellow while the veins stay green due to reduced chlorophyll production. This can stunt plant growth and reduce yield. Iron toxicity, on the other hand, occurs in waterlogged and acidic soils where iron is overly available. Symptoms include bronzing or browning of leaves, necrotic spots, and, in severe cases, plant death. Toxicity is more common in historically waterlogged soils, with acid-related toxicity primarily near mining waste sites.
In certain situations, plants may take up enough iron, but it doesn’t get properly distributed or reach active sites within the plant. Internal iron precipitation, where iron forms insoluble compounds, renders it unavailable for biological processes, leading to deficiencies. This can occur if the internal pH of the plant is too high, causing iron to precipitate and become unavailable for metabolic use, even if the overall iron content seems sufficient on a tissue test. This often occurs in the apoplast, where high pH and oxygen cause Fe3+ to form insoluble compounds.
High internal pH is often observed in calcareous soils, where the plant’s environment has a higher pH. Interestingly, even in soil environments with soluble Fe2+, the plant’s internal environment can promote the oxidation and conversion of soluble Fe2+ to mineral Fe3+.
Along with pH, the precipitation of iron as oxides or hydroxides can occur through Fe2+ interacting with aggressive forms of oxygen, or reactive oxygen species, produced by the plant in response to stressors like drought, nutrient deficiency, or heat stress. These stressors can hinder the production of iron chelators such as organic acids like citrate or specific iron-binding proteins, as they require energy from the plant. Both factors can lead to the precipitation of iron forms that are not available for metabolic processes, resulting in deficiency symptoms.
Certain plant diseases or physiological disorders can also affect the transport or distribution of iron within the plant, leading to localized deficiencies. For instance, in lime-induced chlorosis, often seen in citrus trees grown in high-pH (alkaline) soils, iron may be sufficient in roots but inadequately transported to the foliage, causing deficiency symptoms in the leaves.
Standard tissue analyses typically measure total iron content, including both soluble and insoluble forms. However, the soluble fraction inside the plant is the one readily available and actively participating in metabolic processes. The insoluble “inactive” iron fraction, promoting chlorosis symptoms, is not distinguished in routine chemical analyses. Other tests like plant sap analysis, targeting nutrients within the xylem and phloem, may be a more useful diagnostic tool for predicting iron deficiency, although more research is needed. Weaker, less aggressive soil extracts, such as a saturated paste made up of soil and water, can also determine low iron availability in soils, but these tests require regional calibration based on soil type and climate.
This emphasizes the importance of considering not only the total quantity of iron in a plant but also its form and distribution for proper growth and development.
Iron’s Antagonistic Interactions
Iron’s complex relationship with other soil nutrients is a crucial aspect of plant health. High phosphorus levels can obstruct iron uptake, potentially leading to induced iron deficiency. Similarly, other trace metals like manganese and zinc can compete with iron for uptake, affecting iron’s bioavailability. This emphasizes the importance of not only balanced nutrient management in maintaining optimal plant health and productivity, but also understanding antagonistic interactions in soils.
Armed with an understanding of soil-iron dynamics, farmers can strategically alter soil conditions to promote plant health. For example, adjusting soil pH and aeration can tilt the balance toward the more absorbable ferrous form of iron. Furthermore, the choice of crop varieties can make a difference. Some plants, such as barley, wheat, and maize, have enhanced capabilities for iron uptake and can maintain robust growth even in iron-deficient soils.
Adding another layer of complexity to iron availability is the presence of iron bacteria in waterlogged soils. As discussed previously, iron becomes very soluble and available under oxygen-limited conditions; however, certain microorganisms can metabolize or respire this form of iron, transforming it into forms inaccessible to plants. Despite plants’ remarkable biochemical adaptations for iron uptake, they might find themselves iron-deficient in the presence of iron-hungry bacteria. Thus, understanding and managing the microbial ecology of soils becomes another key factor in maintaining agricultural productivity.
Despite our best soil management practices, there can be circumstances when traditional methods fall short. In these instances, direct iron supplementation to plants can be necessary. One such strategy is foliar iron applications. By bypassing the complex soil matrix and providing iron directly to plant leaves, this approach offers a potential remedial measure against iron deficiency.
Iron and Climate: The Bigger Picture
The role of iron in soils extends beyond immediate agricultural productivity. Both agricultural and wetland soils rich in iron can sequester carbon in stable forms, effectively acting as a carbon sink. In fact, iron minerals, along with finer soil particles (clay and silt), are generally required to grab onto and stabilize soil carbon, keeping it inaccessible to microbes. Understanding and harnessing this interplay between iron and carbon cycles could be a valuable strategy in our fight against global climate change, making iron a potential ally in our efforts to preserve Earth’s climate balance.
Optimizing iron uptake is a matter of global scientific interest, and one that requires a working understanding of soil and plant nutrient management. Along with this, knowing some of the finer details — such as antagonistic interactions between iron and other soil components, including microbes — will help guide some of the more complex problems we may be faced with.
As we grapple with these and other pressing challenges, from crop production to climate change and food security, understanding the complex interactions between nutrients, plants, soils, and microbes will become even more critical. With this knowledge, we can develop novel and sustainable innovations that not only bolster crop yields but also address the broader environmental implications, guided by the understanding that there is still so much more to learn.
Patrick Freeze is a soil health scientist, research and development manager, and technical specialist at Ward Labs. He earned his Ph.D. in soil chemistry from Washington State University, where he studied soil health and heavy metal chemistry as a USDA NIFA Needs Fellow and in Thailand as a U.S. Fulbright Scholar.














