Research scientist Frank Dean talks with John Kempf about chelation, glyphosate and the vast farming knowledge that has been lost
John Kempf: Frank, can you tell us a little bit about your journey as it relates to the agricultural space and some of the things you’ve been working on?
Frank Dean: Well, it started years ago. I worked for Stoller out of college; I put together all his chelates. Then I went to work for LidoChem, which is a chemical distributor. I developed some end-use products that have some value in the marketplace, and I’ve been doing that for over 20 years.
Kempf: I’ve been looking forward to having a conversation with you about chelation. Chelation is often promoted in a positive sense — that we need nutrients that are more chelated and therefore more readily bioavailable. And then, on the flip side, when we talk about the mode of action of various pesticides and herbicides, the mode of action is described as also being a chelation.
So, what is the difference between those two? How is it that on one hand we have chelation being a positive, on the other hand, chelation has the ability to kill plants or other organisms?
Dean: Well, herbicides are able to chelate certain metals. Now, they’re not being distributed on the ground; they’re being distributed on plant tissue, which means that the concentration of metals in the plant is much, much lower than it would be in the soil. So, you have this herbicide, say, going into a plant, collecting all the manganese or iron or copper, and eliminating the ability of the plant to have normal metabolism.
You need these metals — zinc, copper, manganese, iron — as enzyme catalysts inside the plant. If the metals are not there, then the metabolic reactions inside a plant either get slowed down or shut down. And then, as you know, if you have mineral deficiencies in plants, oftentimes the plant becomes very attractive to fungi, bacteria and insects; if the plant has a full load of micronutrients, along with adequate macronutrients, it becomes much more resistant.
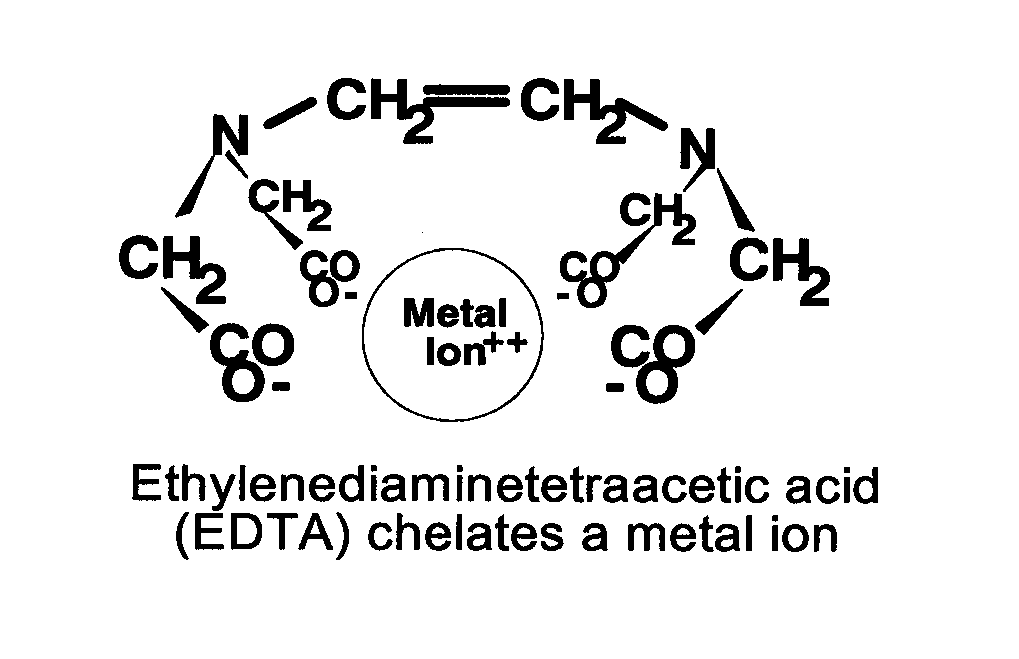
There’s a couple of things we have to understand. The metal is an individual atom. You’ll see them as a little circle on a piece of paper with two little plus signs, which says that they’re positive. Now, a chelate is often a weak acid. As you put it in water or solution, only a little bit of the acid comes off the weak acid, and then that acid has a negative charge. So, if that weak acid can find the metal, then you go from a plus-two to a plus-one. But if there’s more than one weak acid on a molecule, the second arm of the chelating agent attaches much more easily, which means that your original plus-two cation now has no charge at all.
For instance, a metal is an individual atom; EDTA, a chelating acid, has 36 atoms. If you connect two of them, you get no charge at all in the new molecule. If you collect three, that iron or copper begins to take on a negative charge. Things like carbonates, sulfates, phosphates — EDTA can make certain metals insoluble at negative charges.
Now your metal has a negative charge; it becomes immune to attack. So, you have this little round ball of a metal and it’s now being covered by 32 other atoms. Even if the chelate was to fall apart somewhat, there’s still no room for the negatively charged anion to attack the metal. You’ve got this blanket, or web, around the metal, protecting it.
Then, when it’s taken in by the plant, the plant has a pH of, say, 5.5 or 6.0 in its sap. That’s enough to start to break the chelating agent apart and free up the metal. Whether the plant disposes of the chelating agent or metabolizes it, it doesn’t matter at that point. If it were in the soil, the roots would set out organic acids. Those are enough to release the chelating agent and make the metal available. In the plant, sap has to do this.
Now, some herbicides have the same properties. However, the herbicide is shutting down metabolic processes inside the plant.
Support authors and subscribe to content
This is premium stuff. Subscribe to read the entire article.
















