Butterflies and Moths Accumulate Enough Static Electricity to Attract Pollen without Contact
Butterflies and moths collect so much static electricity while in flight that pollen grains from flowers can be pulled by static electricity across air gaps of several millimetres or centimetres.
The finding, published in the Journal of the Royal Society Interface, suggests that this likely increases their efficiency and effectiveness as pollinators.
The University of Bristol team also observed that the amount of static electricity carried by butterflies and moths varies between different species, and that these variations correlate with differences in their ecology, such as whether they visit flowers, are from a tropical environment, or fly during the day or night. This is the first evidence to suggest that the amount of static electricity an animal accumulates is a trait that can be adaptive, and thus evolution can act upon it by natural selection.
The study involved 269 butterflies and moths across 11 different species, native to five different continents and inhabiting multiple different ecological niches. In terms of practical applications, this study opens the door to the possibility for technologies to artificially increase the electrostatic charges of pollinators or pollen in order to improve pollination rates in natural and agricultural settings.
Air Pollution Harms Pollinators More Than Pests, Study Finds
Bees and other beneficial bugs are disproportionately harmed by air pollution compared to crop-destroying pests, a new study published in Nature Communications has found.
Researchers from the University of Reading analyzed data from 120 scientific papers to understand how 40 types of insects in 19 countries respond to air pollutants like ozone, nitrogen oxides, sulfur dioxide and particulate matter. Pollinators — including bees and some moths and butterflies — experienced a 39 percent decline in foraging efficiency after being exposed to elevated air pollution levels. In contrast, plant-eating aphids and other pests were not significantly impacted.
“Air pollution is an underappreciated threat to the insects that make our lives easier,” said lead researcher James Ryalls. “The bees that pollinate our flowers and the wasps that provide natural pest control are at risk of further decline if air pollution levels are not addressed.
The researchers suggest that beneficial insects, such as bees and wasps, are more affected by air pollution due to their reliance on scent-based communication. Many beneficial insects use airborne chemical signals to locate flowers, find mates or hunt their prey. Air pollutants can chemically alter these scent trails or interfere with insects’ ability to detect them, essentially disrupting their sensory landscape. In contrast, many pests rely less on long-distance scent cues and more on direct contact or visual cues, making them less vulnerable to air pollution’s effects on airborne chemical signals.
The study focused on how air pollution impacts various aspects of insect behavior and biology, including feeding, growth, survival, reproduction and ability to locate food sources. Of all these factors, insects’ ability to find food was most severely impaired by air pollution, declining by about one-third on average.
Among air pollutants, ozone emerged as particularly harmful to beneficial insects, reducing their ability to thrive and carry out their roles in the ecosystem by 35 percent. Even low ozone levels — below current air quality standards — caused significant damage. Nitrogen oxides also substantially impaired beneficial insects.
Researchers Find That Frogs Can Quickly Increase Their Tolerance to Pesticides
Although there is a large body of research on pests evolving tolerances for the pesticides meant to destroy them, there have been considerably fewer studies on how non-target animals in these ecosystems may do the same.
In new research, Rensselaer Polytechnic Institute’s Rick Relyea and his team have begun to address this gap in research.
The standard toxicology tests focus on determining the lethal amount of pesticide in one exposure. Relyea and team, on the other hand, first exposed wood frogs to sublethal doses which, in many cases, allowed them to rapidly develop a higher tolerance within a few days.
“In nature, this is the way that animals are likely exposed,” said Relyea. “Animals living near agriculture, lawns, and landscaping, etc. … are likely getting lots of small doses of pesticides. The question is, what does that do to the animals? Our research found that for several commonly used insecticides, wood frogs can increase their tolerance within days.”
Relyea and team examined the tolerance of 15 populations of wood frogs from western Pennsylvania and eastern New York to three common insecticides: carbaryl, chlorpyrifos and diazinon. They compared results when the frogs were first exposed to no pesticide or sublethal concentrations before being exposed to lethal concentrations. The team discovered that nearly half of the populations exhibited rapidly inducible tolerance.
“We think this is a way that animals can more rapidly evolve tolerance to pesticides over many generations,” said Relyea. “If tolerance to a pesticide is first induced by a sublethal exposure, it helps protect the population over generations because that tolerance would be favored genetically. Later generations would not need to be exposed to sublethal doses in order to exhibit increased tolerance.”
It is important to point out that the increased tolerance is limited, however. “We don’t want people to think that pesticides pose no threat to non-target animals,” said Jessica Hua, Ph.D. “They can still die. There is a difference between being more tolerant and being impervious to pesticides. Additionally, this work builds on our research demonstrating that while low levels of pesticides can induce tolerance, this pesticide tolerance can be costly and affect the ability of animals to tolerate other threats, like diseases.”
Pesticide Contamination Is More Than Apple Skin Deep
Pesticides and herbicides can present a safety risk to people who unwittingly ingest them. Protecting human health, therefore, demands sensitive analytical methods to identify even trace levels of potentially harmful substances. Now, researchers have published in Nano Letters the development of a high-tech imaging method to detect pesticide contamination at low levels, and its application on fruits reveals that current food safety practices may be insufficient.
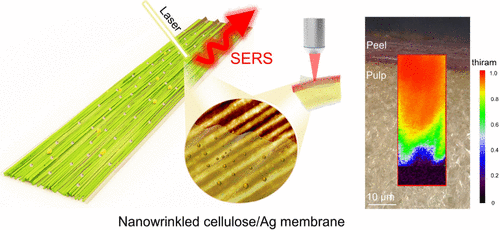
The analytical method called surface-enhanced Raman spectroscopy (SERS) is gaining popularity as a nondestructive method for detecting chemicals from modern farming on produce. With SERS, metal nanoparticles or nanosheets are used to amplify the signals created by molecules when they are exposed to a Raman laser beam. The patterns created by the metal-enhanced scattered light serve as molecular signatures and can be used to identify small amounts of specific compounds. Looking to improve SERS sensitivity for pesticide detection, the researchers designed a metal-coated membrane they could lay atop farm-grown produce. They also wanted to develop the material to be versatile enough to accommodate an array of other applications.
They started with a cellulose hydrogel film, which they stretched to form aligned nanoscale wrinkles along its surface. They then immersed the film in a solution of silver nitrate to coat the grooves with SERS-enhancing silver nanoparticles. The resulting membrane was highly flexible and practically transparent in visible light — essential features for SERS signal detection.
In tests of the silver-embedded membrane for food safety applications, the researchers sprayed the pesticides thiram and carbendazim, alone or together, onto apples, air-dried the fruits, and then washed them, to mimic everyday practices. When they laid their membrane over the apples, SERS detected pesticides on the apples, albeit at low concentrations. The team was also able to clearly resolve scattered-light signatures for each pesticide on apples sprayed with both thiram and carbendazim, as well as to detect pesticide contamination through the fruit’s peel and into the outermost layer of pulp.
These results suggest that washing alone could be insufficient to prevent pesticide ingestion and that peeling would be required to remove potential contamination in the skin and outer pulp.

History Shows That Humans Can Be Good for Biodiversity
Researchers at the University of York utilized a global pollen dataset to help understand the variety of plant communities dating back to around 12,000 years ago, the beginning of the period known as the Holocene. Over this time period, up until the start of the Industrial Revolution, the speed that different types of plants changed within a community accelerated with increased human land use in all continents, suggesting that humans have been an important driver of vegetation change. The research is published in the journal Nature Ecology and Evolution.
However, the team’s analysis of data from pollen records revealed that the nature of these changes varied geographically. Plant communities became increasingly diverse across most of the northern hemisphere, linked to human activity over the period, but in Africa, South America and some parts of North America, increased human land use saw a fall in plant diversity, while locations with more limited human land use saw increases in diversity.
Jonathan Gordon, postdoctoral researcher at the University of York, said, “When we read headlines about threats of extinction to animal or plant life, human activity is often cited as one of the main reasons for the decline. While it is absolutely true that the vast majority of extinctions taking place since 1500 have been driven by humans, over longer time periods the effects of humans on local and regional biodiversity are positive in many areas.”
The study showed that farming and forestry practices interacting with regionally specific plant communities resulted in increased diversity in many of the once-forested areas of the northern hemisphere, where partial clearing of trees to make way for animals, crops and homesteads increased the diversity of habitats and made space for light-loving plants.
“We see a slightly different picture in open grasslands and savannas, compared to forested areas, however, and this could be because it is more challenging for humans to diversify plant life by planting trees, compared with chopping trees down in forested regions,” Gordon said. “In these areas, biodiversity only benefited with less intense forms of human use.”
The research calls for a more varied approach to increasing biodiversity across the globe, with evidence from thousands of years of human interactions with the Earth’s ecosystems being taken into account in new and future environmental policy.
“The common assumption when tackling biodiversity issues is that human influence needs to be removed in order for the environment to thrive as nature intended it to,” said researcher Chris Thomas. “In many places, biodiversity thrives because of many thousands of years of human activities, and in others it can suffer, and so it is important to know the differences in order to develop appropriate conservation policies.”
“In a European context, for example, this work suggests that low-intensity, traditional farming methods practiced over multiple millennia resulted in elevated biodiversity levels,” Gordon added. “Encouraging traditional methods, and reintroducing them in locations where they have now been abandoned, could be part of future conservation strategies that seek to include, rather than reject out of hand, humans from diverse ecological systems.”
Minerals Play Newly Discovered Role in Earth’s Phosphorus Cycle
Northwestern University-led researchers have discovered a new way that nature cycles phosphorus, a finding that uncovers a missing piece of Earth’s puzzling phosphorus cycle. The research was published in the journal Nature Communications.
Although organic forms of phosphorus are abundant in soils, plants and microbes need inorganic phosphorus to spur their own growth. In the organic form, phosphorus is connected to carbon atoms directly or indirectly, using oxygen as a bridge. So, plants and microbes secrete enzymes to break the carbon bond in organic phosphorus to generate bioavailable inorganic phosphorus.
While current understanding of the phosphorus cycle assumes that only enzymes from plants and microbes drive this transformation, the new study shows there is another way. Iron oxide, a naturally occurring mineral in soils and sediments, can perform the reaction that transforms organic phosphorus to generate the inorganic form. Surprisingly, the researchers also found that iron oxide minerals recycle phosphorus at a similar rate as reported for enzymes in soils.
Mined supplies of phosphorus may run out in as soon as 50 years. “We are looking into ways to leverage nature-based solutions for phosphorus recycling,” said Ludmilla Aristilde, who led the work. “But, before we can do that, we need to understand the underlying mechanisms of natural phosphorus recycling. We found that minerals play an important, and previously unknown, role in the process.”
When dead vegetation or microbes decay in the soil, they leave behind a number of nutrients, including DNA and RNA, which are important classes of organic phosphorus. Microbes and living plants use enzymes to cleave phosphorus from nucleotides — structural components in DNA and RNA — in decaying organic matter to make it available as a recycled nutrient. Until now, most researchers assumed using enzymes was nature’s only mechanism for recycling organic phosphorus.
Aristilde and her collaborators, however, decided to explore whether another mechanism might be at play. “Findings from field studies on the environmental dynamics of phosphorus suggested to consider mechanisms beyond biology for the transformation of organic phosphorus in sediments,” Aristilde said.
In laboratory experiments, Aristilde and her team studied the fate of phosphorus in soils and sediments containing iron oxide minerals. After running multiple experiments and analyses, researchers found transformation products from the reaction in the solution. But part of the inorganic phosphorus was curiously missing.
Because iron oxide is known to trap phosphorus, the team wanted to examine the minerals more closely. To do so, they used a specialized x-ray technique at the Stanford Synchrotron Radiation Lightsource to solve the mystery.
“We found that the phosphorus was clinging to the surface of the iron oxide,” Aristilde said. “Basically, the minerals can recycle phosphorus from DNA and RNA molecules. But not all organic phosphorus is released in the solution because it is stuck to the surface. The x-ray technique allowed us to find that a big fraction of the newly generated inorganic phosphorus was associated with iron oxides.”
Aristilde’s team then measured how — and how much — inorganic phosphorus was produced from nucleotides. The researchers discovered that minerals recycle phosphates at a rate comparable to biology.
“We did not expect the rates to be so comparable to those reported for soil enzymes,” Aristilde said. “It changes the way we think about how phosphorus is recycled.”















