Bladder Cells Protect Plants from Pests and Disease

Quinoa and many other extremely resilient plants are covered with strange balloon-like “bladders” that for more than a hundred years were believed to be responsible for protecting them from drought and salt. Research results from the University of Copenhagen reveal this not to be the case. These so-called bladder cells serve a completely different, though important, function: protecting the plant from pests and disease. The finding makes it likely that even more resilient quinoa plants will now be able to be bred, which could lead to the much wider cultivation of this sustainable crop worldwide.
“Quinoa has been touted as a future-proof crop because it is rich in proteins and highly tolerant of drought and salt, and thus climate change. Scientists believed that the secret to quinoa’s tolerance was in the many epidermal bladder cells on the surface of the plant. Until now, it was assumed that they served as a kind of salt dump and to store water. But they don’t, and we have strong evidence for it,” said researcher Michael Palmgren.
The researchers cultivated mutant plants without bladder cells to compare their reactions to salt and drought with those of wild quinoa plants covered with bladder cells. Epidermal bladder cells are fluid-filled hair structures on the leaves, stems and surfaces of a variety of plants. A few plants, including quinoa, are often completely covered with them. Bladder cells are actually a form of trichomes. Trichomes are hairlike structures that most plants have.
In 1896, Austrian plant physiologist Gottlieb Haberlandt proposed that bladder cells serve as water reservoirs. The number of bladder cells on a plant is predetermined. A very young leaf has the same number of bladder cells as an old leaf, but the density on the young leaf is greater because of its smaller size. As such, young leaves, which are most attractive to pests, are better protected.
Bladder cells are found in members of the Amaranth family (e.g., quinoa and white goosefoot) and the Aizoaceae family (including ice plant), which combined include approximately 3,840 species worldwide.
To their surprise, the researchers discovered that bladder cells have no positive influence on the plant’s ability to tolerate salt and drought. On the contrary, they seem to weaken tolerance. Instead, bladder cells serve as a barrier against pests and disease. “Whether we poured salt water on the mutant plants without bladder cells or exposed them to drought, they performed brilliantly and against expectations. On the other hand, we could see that they were heavily infested with small insects — unlike the plants covered with bladder cells. That’s when I realized that bladder cells must have a completely different function,” said Max Moog, first author of the study, which has been published in the journal Current Biology.
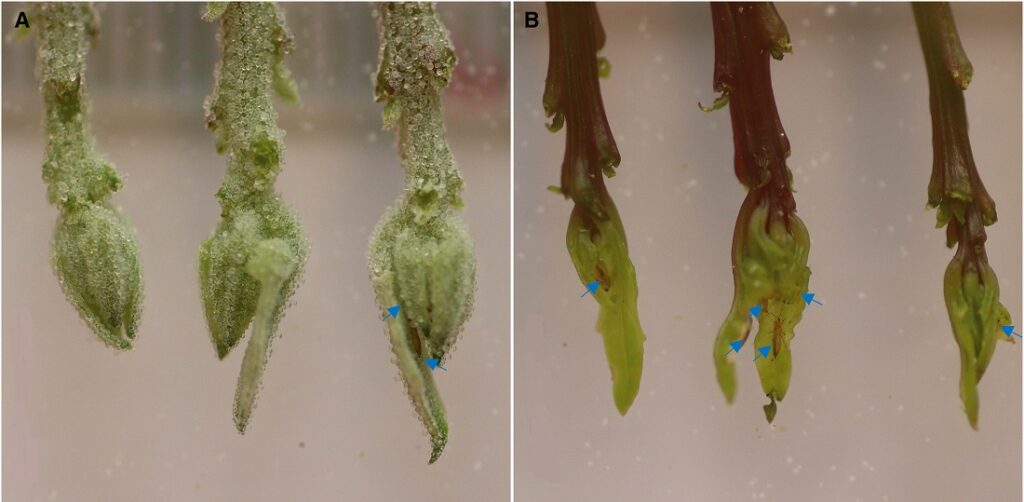
When the researchers analyzed what is hidden inside the bladder cells, they did not find salt as expected — despite having added extra salt to the plant. Instead, they found compounds that repel intruders.
“We discovered that bladder cells act as both a physical and chemical barrier against hungry pests. When tiny insects and mites trudge around on a plant covered with bladder cells, they are simply unable to get to the juicy green shoots that they’re most interested in. And as soon as they try to gnaw their way through the bladder cells, they find that the contents are toxic to them,” said Palmgren.
Among other things, the epidermal bladder cells of quinoa contain oxalic acid, a compound also found in rhubarb, which acts as a deadly poison to pests.
The experiments also demonstrated that the bladder cells even protect quinoa against one of the most common bacterial diseases in plants, Pseudomonas syringae. This probably happens because the bladder cells partially cover the stomata on the plant’s leaves, a point of entry for many bacterial invaders.
“Our hypothesis is that these bladder cells also protect against other plant diseases like downy mildew, a fungal disease which severely limits quinoa yields,” said Moog.
There are thousands of varieties of the South American crop, and the density of bladder cells on the plant’s surface varies from variety to variety. But there is much to suggest that density determines how effective a safeguard the bladder cells are.
According to Palmgren, the new results provide a concrete recipe for how to breed “super-quinoa” relatively easily. “Thus far, these bladder cells have been ignored in the breeding of quinoa. If you want a crop that is extra resistant to pests and diseases, but is still tolerant of salt and drought, one can opt to breed varieties that are densely covered with bladder cells.
Adding Calcium to Soils Can Help Increase Organic Matter, Trap More Carbon
Farmers add calcium to their soil for many reasons, including regulating pH and improving soil structure.
Using the Canadian Light Source (CLS) at the University of Saskatchewan, scientists from Cornell University and Purdue University have identified a previously undiscovered mechanism triggered by calcium when it’s added to soil. Their finding could lead to more strategic use of the mineral in agriculture.
Researchers already knew that calcium impacts the way organic matter is stabilized in soil. What wasn’t known was whether calcium had an effect on which microbes were involved and how they acted. Microbes are microscopic organisms that live in the air, soil, and water; in soil, they process soil organic matter and help promote plant growth.
“We showed that by adding calcium to soil, we changed the community of microbes in the soil and the way they process organic matter,” said lead researcher Itamar Shabtai. “They processed it in a more efficient manner — more carbon was retained in the soil and less was lost to the atmosphere as CO2.”
Carbon, which makes up about half of the organic matter in soil, is incredibly important to almost all soil properties. “Soils that contain more carbon are generally healthier,” said Shabtai. “They are better able to hold on to water in drought conditions. Soils with higher amounts of organic carbon are also able to deliver nutrients more efficiently to plants and promote plant growth, and they’re more resistant to erosion.”
The researchers used the SGM beamline at the CLS to measure the amount of plant matter decomposed following the addition of calcium, while the mid-IR beamline enabled them to identify and quantify the stabilized carbon in the soil — data impossible to gather in any other way.The findings were published in Nature Communications. This press release was produced by Canadian Light Source (lightsource.ca).
Zapping Manure with Special Electrode Promises an Efficient Method to Produce Fertilizers, Other Chemicals

An interdisciplinary team led by University of Wisconsin-Madison scientists has developed a new technique that could help farmers extract useful nutrients such as ammonia and potassium from livestock manure to efficiently make fertilizer and other useful chemical products.
While the strategy still needs to be scaled up beyond a proof-of-concept stage, the group’s preliminary analyses show it could offer considerable benefits by cutting water and air pollution while simultaneously creating products that farmers could use or sell.
Manure stinks in part because it contains ammonia, one of the more than 300 compounds that contribute to its odor. The pungent gas is not only a harmful air pollutant but can turn into the greenhouse gas nitrous oxide and water-polluting nitrate.
Ammonia is also the foundation of many nitrogen fertilizers that have fueled modern crop production. The industrial method for making ammonia for nitrogen-based fertilizers, the Haber-Bosch process, consumes a lot of energy and emits hundreds of millions of tons of greenhouse gasses every year.
Although manure itself can be used as fertilizer, doing so can be costly and logistically challenging and has environmental drawbacks. So, researchers around the world are hunting for strategies to efficiently recover ammonia from manure, creating more concentrated and valuable fertilizers that are greener and more affordable to transport.
Among these strategies are chemical processes driven by electricity, which is becoming increasingly inexpensive in many rural communities thanks to growing solar and wind power generation. However, most electrochemical techniques in development are not yet practical, mainly because they consume a lot of energy and aren’t efficient enough at pulling dissolved ammonia (in the form of ammonium ions) out of manure.
This new technique, described in the journal Nature Sustainability, relies on a specially designed electrode, like those used for batteries, that targets ammonium ions.
The technique involves a nickel-based electrode that is placed directly into the manure wastewater. As organic matter in the manure naturally gets oxidized by the electrode, the ammonium, as well as potassium ions, within the wastewater are selectively driven into and captured by the electrode.
The strategy does not end with simply removing the nutrients from the wastewater. In an innovative step that could help make the process even more attractive, the nutrient-loaded electrode is then placed into a device that uses electricity to release the recovered ammonium and potassium ions, which can then be used to make nitrogen and potassium-based fertilizers, and simultaneously to produce other useful chemical products. These could include hydrogen fuel or hydrogen peroxide, which is commonly used for disinfection.
Trial runs with small amounts of manure recovered more than half the ammonia in the first pass, with a recovery of about 85 percent after two cycles.
The ability to produce fertilizers and other chemical products together is a key part of why the team believe their strategy could be a winner. An environmental analysis led by Rebecca Larson, a professor in the Nelson institute for Environmental Studies, indicates that a 1,000-head dairy farm operation could reduce its ammonia emissions by more than 50 percent by deploying the system, while also significantly reducing the amount of nitrate entering nearby waters.
Meanwhile, a preliminary technical economic analysis led by professor Fikile Brushett, a collaborator at Massachusetts Institute of Technology, shows that a model dairy farm using the system could expect resulting revenues to be higher than operating costs, so long as electricity prices aren’t exorbitant.
The next steps include further improving the materials and processes, scaling the system up and studying how it functions at a level more closely resembling a real-world livestock operation.
New Method Turns Mine Waste into Healthy Soil
Tailings — the waste left after extracting precious and critical minerals — often contain harmful chemicals and heavy metals that can pollute soil, water and crops. There are over 1,800 tailings storage facilities around the world, and in 2019, a tailings dam in Brazil collapsed; close to 300 people drowned in the waste, which also polluted local land and waterways.
Now a team led by researchers at the University of Queensland has developed an innovative method to turn harmful tailings into healthy soil. The scientists used the Canadian Light Source (CLS) at the University of Saskatchewan to determine the underlying mechanism of their process. Their findings were published recently in Environmental Science & Technology.
It’s costly and environmentally risky to store tailings over the long term, and other processes for remediating mine waste are slow and extremely expensive. “We have basically taken engineering solutions into the context of natural soil formation from rocks, because tailings have some useful minerals common to natural rocks,” said lead researcher Longbin Huang. The team’s solution, he said, could save billions of dollars around the world and carry a host of environmental benefits.
“Tailings have no biologically friendly properties for growing plants. Roots and water cannot penetrate them, and soluble salts and metals in tailings can kill plants and soil microbes,” said Huang. “If you wait for nature to slowly weather the tailings and turn them into soil, it could take a couple thousand years.”
Huang and colleagues found a way to accelerate natural soil formation processes to convert tailings into healthy soil. “We can convert these colossal volumes of biologically hostile tailings into growth media similar to natural soil by developing soil structure that will enable biological activity of microbes and plants, basically establishing a natural ecosystem,” he explained.
The process involves encouraging specific microbes to grow in tailings that have been amended with plant mulch from agricultural waste and urban green waste. These microbes “eat” the organics and minerals in tailings, transforming them into functional aggregates (or soil crumbs), the building blocks of healthy soil.
“You have microbially active surfaces in soil crumbs that develop a porosity in compacted tailings that allows the gas, water, roots and microbes to survive, just like in arable soil. Therefore, the dead mineral matrix of tailings becomes a soil-like media that will enable plants to grow.” Huang noted that this process — which can occur in as little as 12 months — can also be used to restore soils damaged by over-farming, overuse of fertilizers and climate change.
Using the CLS’s synchrotron light, the scientists could visualize the detailed mechanism of how they were able to develop the organic-mineral interfaces and revitalize the tailings. “We needed to use the SM beamline to unravel at the nanometer scale the immediate interfaces and how the minerals change, and how they interact with organics,” said Huang.
The team has also completed a field trial and an extensive greenhouse trial using the rehabilitated tailings to grow crops and native plants.
“We are confident that it works. The maize and sorghum love it!” he said. “The technology is usable now. Someone just needs to use it at mine sites.” The researchers are currently looking for industrial partners.
The research group is hopeful that their method could significantly reduce costs and risks from mining activities, environmental contamination, and even the amount of fertilizer needed by farmers.
This press release was produced by Canadian Light Source (lightsource.ca).












