Through the rhizophagy process, plants can accumulate complex molecules like fatty acids — which improve their health and resilience — from microbe cell walls
Plants accumulate nutrients in several different ways. Two of these — mycorrhizal root colonization and rhizophagy — have become recognized as particularly important. When these systems function at optimal levels, their cascading and compounding effects do more than supply simple nutrients to the plant: they actively drive and maintain plant health. We have recently discovered, in fact, that through these processes, plants are able to accumulate even large, complex molecules such as fatty acids, which significantly contribute to plant health.
Maximizing the energy plants capture is only possible when there is a good root association with both fungi and bacteria. Healthy plants always have very high photosynthetic efficiency values and high amounts of stored energy.
Mycorrhizae literally translates to “fungus-root.” The plant and the fungus have a mutually beneficial relationship in which the fungus facilitates water and nutrient uptake in the plant and the plant provides food and nutrients, created by photosynthesis, to the fungus. This exchange is a significant factor in nutrient cycles and in the ecology, evolution, and physiology of plants.
“Rhizophagy” means “plants eating microbes.” It is a process in which bacteria and fungi cycle between a free-living phase in the soil and a plant-dependent phase within cells of plant roots. Inside the root tip, the bacterial cells located within the periplasmic spaces of the root cells convert to cell-wall-less protoplast forms because the plant’s reactive oxygen degrades the cell walls, effectively extracting nutrients from microbes. Plants attract beneficial microbes to their rhizosphere by producing the exudates they feed on. Microbes obtain nutrients (nitrogen and minerals) in the soil, and the plant then extracts these nutrients.
Increased Lipid Synthesis
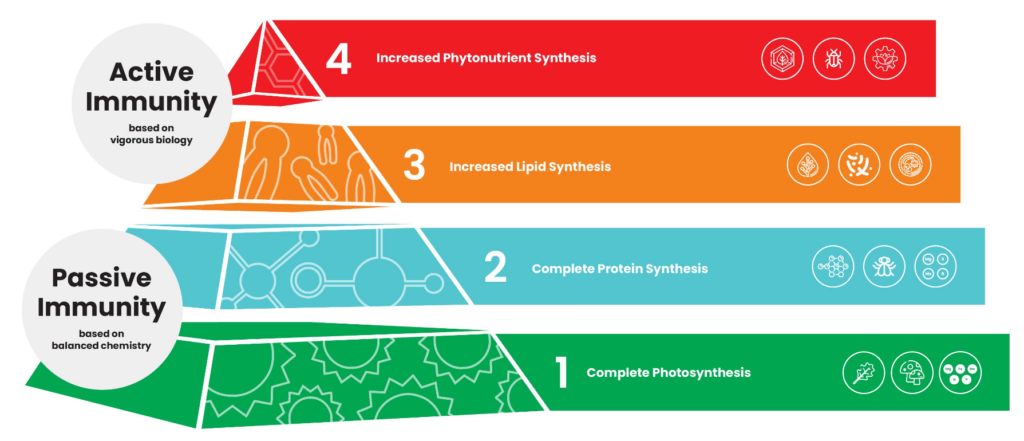
The plant health pyramid, as developed and explained by John Kempf, is a helpful rubric for understanding how plants can become resistant to disease and pests.
The first level of the pyramid is complete photosynthesis. Crops that have a strong association with mycorrhizae and rhizophagy have high photosynthetic activity because of the increased demand by the fungi and bacteria for carbohydrates. Microbes help the plant accumulate the nutrients it needs to optimize photosynthesis.
In the second level — complete protein synthesis — nitrogen is converted into amino acids, and amino acids are converted into proteins. Crop plants that have strong mycorrhizae and rhizophagy have immediate, on-demand access to the mineral nutrients that are essential cofactors for the enzymes that facilitate this synthesis: Fe, Zn, Mn, Mg, S, Mo and B.
Yet it is in the third level — increased lipid synthesis — that mycorrhizal colonization and rhizophagy really begin to improve plant health.
Plants are capable of absorbing relatively complex compounds from the soil profile. John Kempf calls these “prefabricated parts,” and they save the plant a tremendous amount of energy. We have found this to be true through the process of rhizophagy.
The rhizophagy cycle plays an important role in aiding in the synthesis of triglycerides (a neutral lipid fatty acid — NLFA). We uncovered this phenomenon when we compared 15 corn inbred hybrids from Dr. Walter Goldstein of the Mandaamin Institute (read the interview with Dr. Goldstein on page __ of this issue!) with 12 conventional lines. The corn lines bred by Dr. Goldstein were selected for nitrogen-use efficiency and have a strong relationship with the soil microbiome.
We did complete phospholipid fatty acid (PLFA) and NLFA analyses on the root samples of all the cultivars in the comparative trial. We also did PLFA and NLFA analysis on the leaves of these lines. Our initial objective was to establish the level of mycorrhizae colonization and to get an indication of the general microbiological status of the roots.
NLFA analysis of mycorrhizal biomass (ng/g)
| Corn Type | High | Low | Average |
|---|---|---|---|
| Mandaamin Institute corn | 231,929 | 6,673 | 67,391 |
| Conventional corn | 95,104 | 4,783 | 29,895 |
The data clearly confirms more mycorrhizal colonization with the Mandaamin cultivars as compared to the conventional ones.
The other phenomenon that surprised us was the fact that the PLFA root analyses exhibited significant differences in the endophytic gram-negative bacteria biomass.
Gram-negative bacteria NLFA/PLFA ratio
| Corn Type | High | Low | Average |
|---|---|---|---|
| Mandaamin Institute corn | 43.8 | 7.4 | 28.4 |
| Conventional corn | 3.3 | 0.7 | 1.9 |
One consistent observation was a low gram-negative bacterial biomass as measured with PLFA in the roots of the Mandaamin lines. The reduced gram-negative biomass of the Mandaamin lines compared to the conventional lines is a good indication that the exposure to the reactive oxygen in the rhizophagy process decomposes these cell walls very rapidly, leading to low measured biomass. When we look at the NLFA, which represents the triglycerides, we find reasonably high values on both conventional and Mandaamin lines.
NLFA primarily represents storage lipids, such as triglycerides, which bacteria typically do not produce in large quantities. Since both gram-positive and gram-negative bacteria are limited in their ability to store these lipids, bacterial NLFA levels would be expected to remain consistently low across different samples, independent of rhizophagy activity.
Any triglycerides found in association with bacterial structures might instead reflect secondary uptake from other lipid sources or decomposition products, rather than active synthesis by the bacteria themselves.
As rhizophagy degrades gram-negative cell walls and membranes, released lipopolysaccharides and fatty acids might undergo further transformation, potentially leading to the detection of these lipids in NLFA forms. This would increase the NLFA/PLFA ratio specifically for gram-negative bacteria in high-rhizophagy samples, as PLFA from intact gram-negative bacteria decreases and NLFA from their decomposed lipid byproducts becomes more detectable. This is exactly what we found:
Leaf bacterial gram-negative NLFA/PLFA ratio (triglyceride leaf storage)
| Corn Type | High | Low | Average |
|---|---|---|---|
| Mandaamin Institute corn | 5.28 | 0.038 | 2.28 |
| Conventional corn | 0.256 | 0.031 | 0.2 |
We did a few leaf samples and found the same phenomenon as in the roots.
Breaking Down Cell Walls
This initial work opens a new and exciting area for research.
It is unknown exactly which nutrients are transferred to the plant via rhizophagy and how important this process is for nutrient acquisition. We do not know exactly what happens to the cell wall components of the bacteria that are decomposed during rhizophagy. But we do know that these cell walls are rich in phospholipid fatty acids and lipopolysaccharides.
We pose the following hypothesis:
- Bacterial cell walls contain phospholipids and lipopolysaccharides that, when broken down, release fatty acids and carbon skeletons. These can enter the plant’s metabolic pathways and potentially be reassembled into various lipid forms, including triglycerides, which serve as energy storage.
- After internalizing and degrading the bacteria, plants can repurpose the fatty acids from bacterial membranes for their own metabolic needs. Plants may either store these as triglycerides in lipid droplets or use them in membrane synthesis for new cell growth, especially under conditions in which nutrient recycling is advantageous.
- The carbon compounds from decomposed bacteria could also act as precursors in the plant’s own fatty acid biosynthesis pathways. This provides building blocks for energy storage or membrane lipids.
- In some cases, plants with active rhizophagy have shown increased lipid content in roots, which may include triglycerides. These energy reserves are important for root development, stress responses and supporting growth processes like photosynthesis.
- While bacterial cell walls don’t directly convert to triglycerides, the fatty acids and carbon skeletons they provide can enter plant lipid metabolism, potentially contributing to the synthesis of triglycerides and other energy storage forms within the plant.
The fact that we have found such significant differences between conventional corn lines and those bred for soil microbiome association is further proof that many conventional corn cultivars have lost some of their ability to associate with the soil microbiome and would therefore not be suitable for incorporating into a regenerative cropping system. The intensity of this loss differs from cultivar to cultivar, as can be seen from the quantified ranges within the conventional and Mandaamin lines.
We are planning a large-scale trial during the upcoming season to compare corn cultivars for their ability to associate with the soil microbiome. If you are a grower or seed producer interested in participating in this trial, please contact the author.
The participating team that made this initial work possible includes Dr. Walter Goldstein of the Mandaamin institute, Dr. James White from Rutgers university, Dr. Laura Kavanaugh from Advancing Eco Agriculture and Willie Pretorius from Ward laboratories and Reconstruct-Ag.
Willie Pretorius is a soil health consultant with degrees from the Universities of Pretoria and Stellenbosch.















